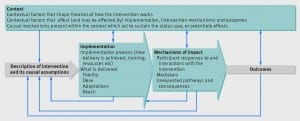
Yesterday Professor Alicia O’Cathain from the School of Health and Related Research at the University of Sheffield discussed the Medical Research Council (MRC) guidance on process evaluation published in the BMJ in 2015 (https://www.bmj.com/content/350/bmj.h1258). In a talk on ‘Using process evaluations alongside randomised controlled trials and other outcome evaluations’, Professor Alicia O’Cathain identified the key recommendations for process evaluations by using the example of a process evaluation alongside a pilot randomised controlled trial and a process evaluation alongside a fully-powered randomised controlled trial of a complex intervention to improve adherence to medication in adults with cystic fibrosis.
Professor Alicia O’Cathain described how context, implementation and mechanisms are the key functions of process evaluations and how an understanding of the interaction of these functions can inform the interpretation of outcomes in randomised controlled trials. It was great to hear about her experiences through different stages of process evaluations, including the planning, design and conduct, analysis and finally the reporting of the outcomes from these evaluations. In addition, it was useful to hear examples of how the results from process evaluations can be used to adapt the delivery of interventions within feasibility studies.
It was extremely beneficial to have the chance for questions and discussion with Professor Alicia O’Cathain and we look forward to using the MRC guidance in our ongoing and future research.
Thank you for this interesting and informative talk!